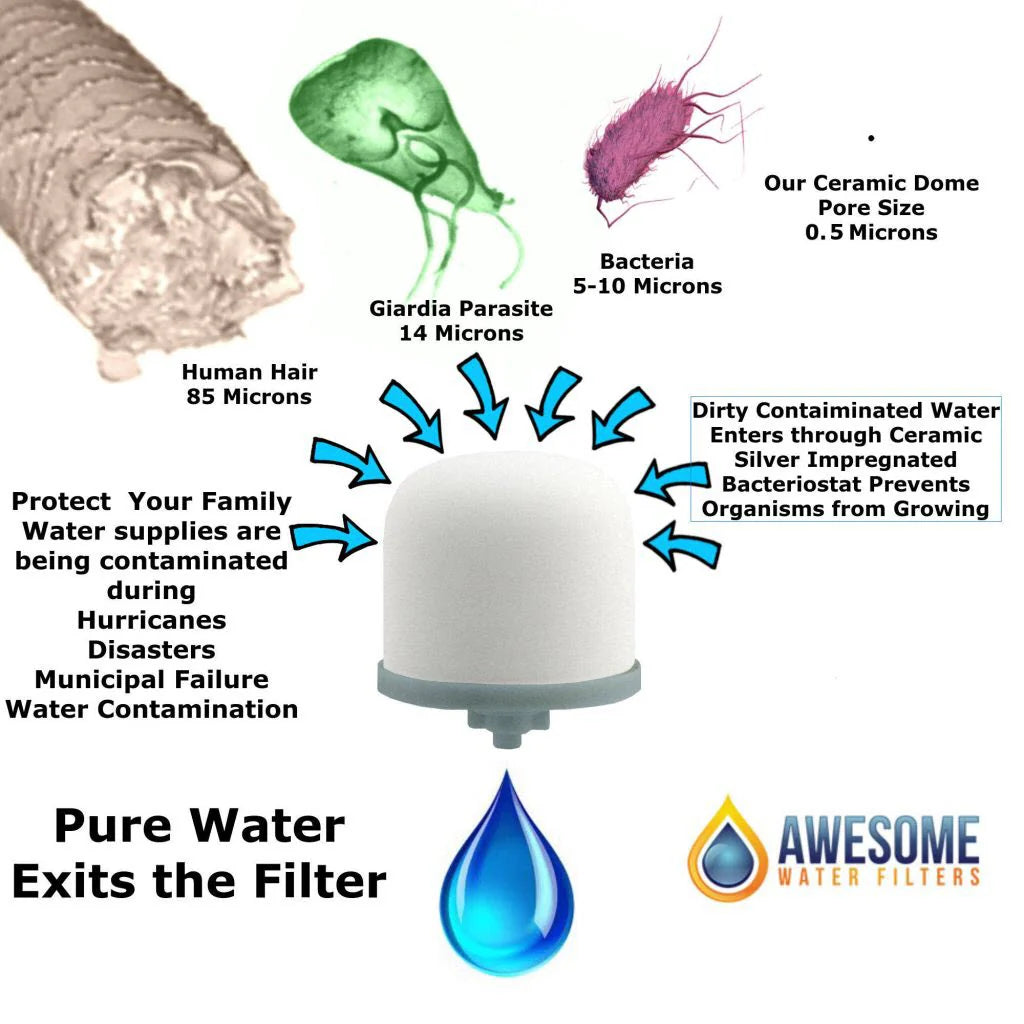
Product and service amazing.
My 1 star rating is invalid. The dome is fine. Sometimes when the water level reaches the 8 stage filter, it somehow causes the flow to restrict. It takes some problem solving, but it works fine other than needing to be cleaned every couple of hours
It only lasted 2 days. And now there's no flow whatsoever. I scrubbed with a scour and rinsed under running water like the instructions said, and little by little the holes got blocked up and now I just WASTED MY MONEY
Quick and easy from buying to delivery thanks